Announcing, our latest feature rollout: Digital Signatures.
The feature allows organisations to move from impractical and inefficient paper signatures and audit trails to simple and trusted Digital Signatures.
What is a Digital Signature?
A Digital Signature is an electronic fingerprint that associates a user with a recorded transaction. It is primarily used in pharmaceutical, drug, medical, and biotechnology organisations that do business through the Food and Drug Agency (FDA) or Medicines and Healthcare products Regulatory Agency (MHRA), who fiercely regulate how information is managed, shared and audited.
In the context of businesses moving from paper signatures to electronic signatures, Hark’s new feature enables organisations to track, attribute, and audit securely in line with FDA 21 CFR part 11:
- Security – the right people, having the right access, at the right time
- Attribution of work – the ‘who, what, when, where and why’ of work performed
- eSignatures – includes ID of the signer, their full name, the date and time of the action, and the reason (updating/deleting/creating)
In practice, this means that if an organisation has the ‘Digital Signatures’ feature switched on, and a logged-in user performs an action (i.e. changing an alert boundary on a sensor) they will be required to re-authenticate before the action can be executed. If all is correct, the action follows through and a Digital Signature will be created. If the entered details are incorrect, an error will be displayed and the action will not complete, thus protecting information from changing with no attached responsibility.
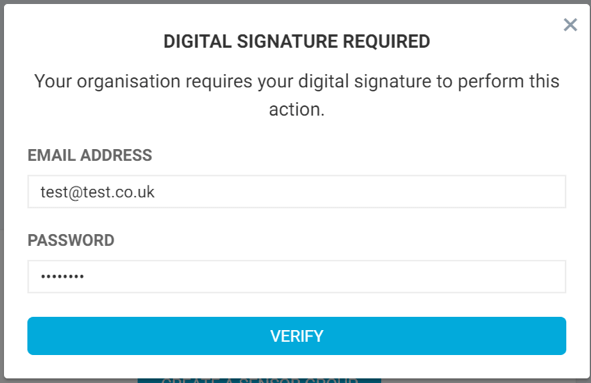
Our Platform
The Hark platform has over 50 actions that require a Digital Signature for such organisations, and this new feature will allow them to ensure the correct level of security around their information, increase efficiency, reduce human errors, and can be counted as equivalent to paper records.
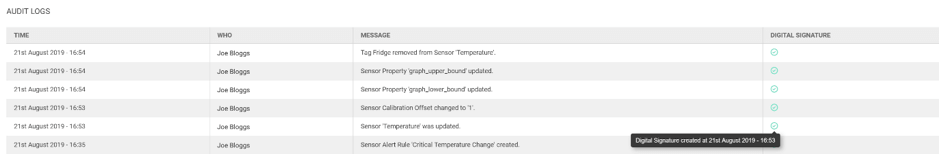
Security for our clients is a top priority at Hark, and by adding our Digital Signatures feature, our heavily regulated customers now have the ability to sign electronically and prove their compliance. If you’re interested in testing this service, get in touch with a member of our team.



