Home › Solutions
Energy Management Analytics and Control Solutions
Our energy management software allows C&I users to connect, analyse and control their energy asset portfolio. From Solar PV to Battery Storage and Sub-Metering.

Enabling Energy Managers and Asset Operators to Digitise, Maintain and Optimise Their Estate.
We are helping alert C&I Energy Managers and Asset Operators about anomalies across their estates.
Informing actions such as operational maintenance, control strategies, asset control and energy management.
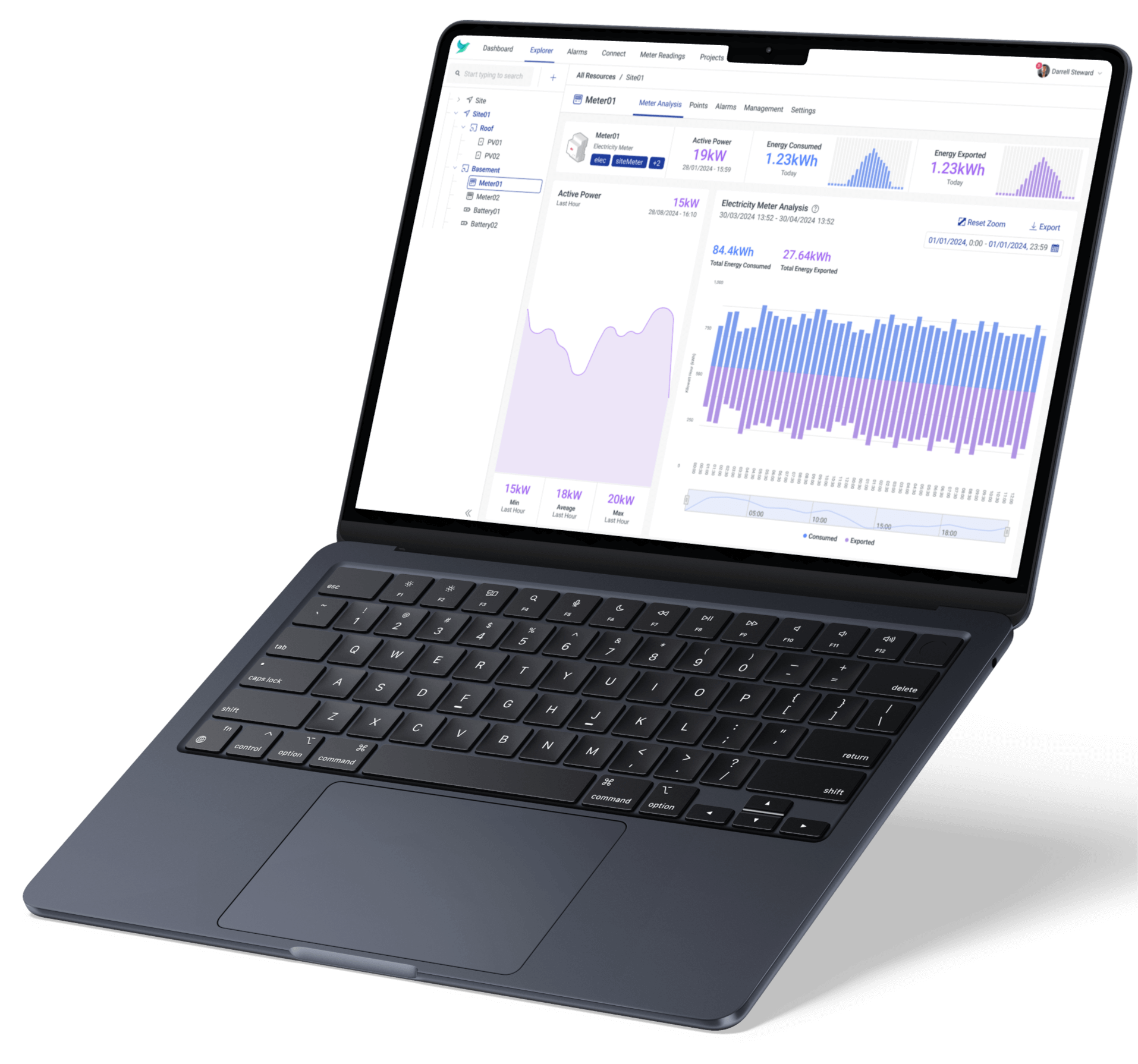
Our Solutions
Enable real-time device and asset monitoring, agnostic of vendor and type. By supporting enterprise, industrial and SCADA protocols, enterprises have a unified view across their estates.
Energy analytics and control. Real-time energy and asset data in a single system, with custom dashboards and automations.
Monitor, control and optimise solar PV, and other energy assets, with unprecedented precision.
Use advanced energy control strategies to revolutionise energy networks across your estate.
Create automated energy control strategies to optimise energy performance throughout your estate.
Peak shaving programs will automatically reduce energy consumption at peak times, resulting in higher efficiency, lower costs and reduced strain on the grid.
Effortlessly become G100 compliant with Hark’s type-tested export limitation device.
Connectivity Support for the Edge or the Cloud
From edge to cloud, Hark technology creates a unified approach to data collection, management and visualisation.
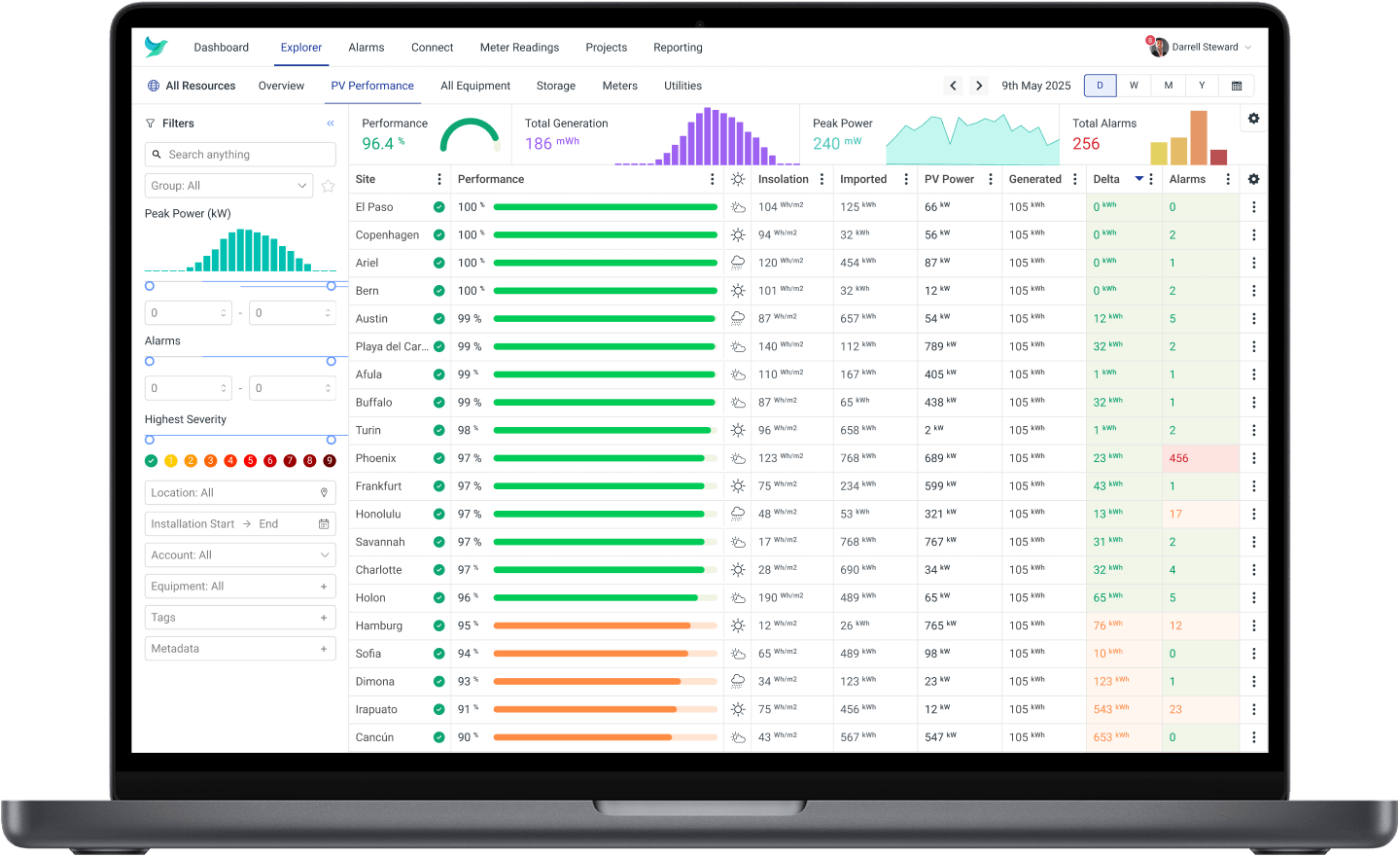
Trusted by Enterprises
We help the world’s leading organisations connect to and monitor their estates, buildings and assets.

“Software is easy to deploy and ahead of the game when it comes to demand response functionality.”
Amy Cluley

“Easy to use and really clean views. The software is really impressive in terms of the variety of widgets that can be used to display and help analyse the data.”
Chris Henderson
Flexible Capabilities and Use Cases
Capabilities
With the power of The Hark Platform, the possibilities are endless. Here are some of the things you can monitor, record and optimise with our advanced technology…
Energy & Power Monitoring
Solar PV Monitoring
Battery Energy Storage (BESS) Monitoring
EMS and Energy Control Strategies
Asset Control and Optimisation
Fault and Status Monitoring
Use Cases
See how we’ve helped market-leading organisations massively increase efficiency and reduce waste by implementing Hark IoT software into their estates.
Simplify Your Route to Smart, Optimised Energy Today!
Take control of the energy transition and see what our energy management software can do for your portfolio.
Getting Started With The Hark Platform
Integrating energy management software into your estate isn’t as tricky as you might think. In three steps you can achieve a fully connected and automated estate that provides pertinent data and enables total optimisation.
Connectivity
The first step involves hardware that provides connectivity to energy and industrial devices, assets and sensors on the edge and if required stream that data into the cloud.
Configuration can be managed by an easy-to-use web interface whether deployed on-premises or in the cloud. The benefits of this include:
- Remote energy monitoring
- Energy management
- Secure device and asset connectivity
Visibility
The second step is visualising your data, in a way that allows you to proactively maintain assets, spot important patterns and ultimately begin to optimise your estate.
Our hierarchy and dashboard features empower the visualisation and analysis of data from industrial assets, energy systems, and other sensors to monitor and report in real-time. This step will empower:
- Operational maintenance.
- Data aggregation and energy reporting.
- Energy Management and Control Strategies
Find out more about our Energy Management Software Load Insights.
Intelligence
The final step to having a fully optimised smart estate is intelligence. Now that your assets are all connected, and your data is streaming into an intuitive platform, the real magic can happen.
Things like smart triggers and alerts, and automated actions are now enabled, meaning the next time an asset under-performs, we can automatically adjust its output and alert the right engineer to look at it. Now you can save costs and carbon by shutting off unused assets and resources.
- Automated and configurable events, rules and controls.
- Real-time triggers and alerting.
Want to Connect Your Assets?
Let’s talk about how energy management software could seamlessly integrate into your organisation, just get in touch today.